Abstract
Introduction
Advances in cancer diagnosis, treatment and supportive care have contributed to improvements in overall survival (OS) in recent decades. Granulocyte colony-stimulating factor (G-CSF) has been shown to reduce neutropenic complications associated with chemotherapy. G-CSF support may improve disease control and survival by minimizing dose reductions and treatment delays as well as enabling administration of dose-dense regimens, dose escalation and additional chemotherapeutic agents when indicated. With the introduction of biosimilar G-CSFs, the impact of G-CSF on OS reported in recent randomized clinical trials is of considerable interest. We undertook an updatedsystematic literature review to determine the impact of G-CSFon delivered dose intensity and OS in cancer patients receiving chemotherapy for solid tumors or malignant lymphoma.
Methods
Following our previous review (Lyman G et al, Ann Oncol 2013;24:2475-84), relevant publications between September 1, 2012 and March 20, 2017 were identified according to best-practice principles and reported in compliance with PRISMA requirements. Randomized, controlled trials comparing primary G-CSF support vs no initial G-CSF and reporting survival measures or occurrence of secondary tumors including acute myeloid leukemia [AML] or myelodysplastic syndrome [MDS]) with a minimum follow-up of 24 months were identified. Following the initial treatment cycle, control subjects may have received no G-CSFs, G-CSFs at the discretion of the treating physician, or secondary use of G-CSFs. Exclusion criteria included non-random assignment to the treatment group; G-CSF administered from the start in all study arms; use of granulocyte macrophage colony-stimulating factor; patients receiving stem cell or bone marrow transplantation, or a prior diagnosis of leukemia of any type.
Full electronic search was undertaken of of Embase, Medline In-Process and Cochrane libraries. No language restrictions were imposed on the literature search. Abstracts of papers initially identified were evaluated independently by two investigators for eligibility using specified inclusion/exclusion criteria. All potentially relevant papers were evaluated in detail. Bibliographies of all identified relevant studies were used to perform a recursive search. Full papers were assessed independently by two investigators according to the predefined eligibility criteria. Any disagreement between investigators was resolved by discussion.
A meta-analysis was conducted to estimate the relative risk (RR) and risk difference for OS between patients who did or did not receive primary G-CSF support. As previously reported, four prospectively-defined study designs reflecting differing dosing schedules were evaluated for impact of primary G-CSF support.Statistical software R was utilized for these analyses.
Results
A total of2604 publications were identified. After removing duplicate reports and further screening, 14 recent studies met the inclusion criteria. Nine studies reported OS and 2 reported incidence of AML/MDS. Sample sizes ranged from 85 to 2091 patients with solid tumors or lymphoma at various stages.
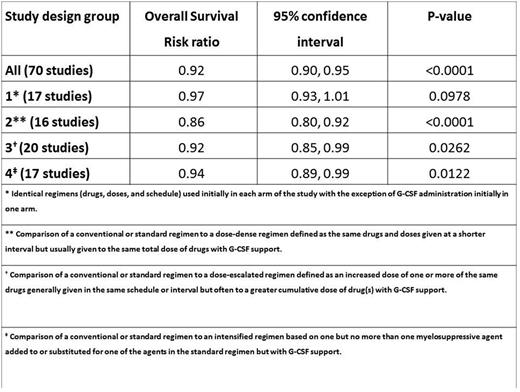
The updated meta-analysis showed that OS was significantly improved in patients receiving primary G-CSF vs patients who did not (relative risk [RR] of death during follow-up = 0.92; 95% confidence intervals [CI]: 0.90, 0.95; p<0.0001) [Table]. Study design groups 2, 3 and 4 also had significant RRs favoring G-CSF (see table). Based on the reported and extracted data, it was not possible to make any conclusions regarding OS differences between originator and biosimilar G-CSF formulations.
Of the 2 studies evaluating AML/MDS, 1 reported no cases during follow-up in either arm and the other reported 9 occurrences in the G-CSF arm and 2 in the control arm: RR = 1.85; 95% CI: 1.19, 2.88; p=0.0064.
Conclusions
Results of this systematic literature review and meta-analysis confirm a modest but statistically significant increase in OS in patients undergoing chemotherapy with primary G-CSF support for solid tumors and lymphoma.
Yau: Hexal: Employment. Nakov: Hexal: Employment. Krendyukov: Hexal: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal